Fig. 1: The synovial membrane in vivo.
Inflammatory, degenerative joint diseases are the leading cause of musculoskeletal pain and dysfunction worldwide. Advanced stages, in particular, are associated with impaired physical activity and chronic pain and the associated mental strains on patients, and the economic burden on society. Given the demographic change and the increase in life expectancy, a further increase in joint diseases is expected. Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease primarily affecting the joints maintained by three distinct pathomechanisms: (i) autoimmune-mediated sterile synovitis, (ii) joint destruction due to tumor-like proliferation of synovial tissue (pannus formation), and (iii) extra-articular destruction. The inflamed synovial membrane was identified as a critical component.
Given the unmet medical need, a clinical and preclinical demand for action derives from increased research in the field. Animal models (primarily mice and rats) are the gold standard in preclinical research to delineate pathogenesis. Alternative methods in arthritis research that allow complex synovial membrane pathologies to be modeled are currently scarce. So far, mainly simplified 2D models have been described, which do not represent the natural physiological tissue architecture. Therefore, 3D models are progressively developed, but these models are primarily based on cell lines and animal matrices.
The project aims to develop and validate a xeno-free, human-based, in vitro 3D synovial membrane model to simulate the pathomechanisms of arthritis - synovitis and synovial proliferation (pannus formation) - to promote preclinical research along the 3Rs' lines. Our model will reflect the physiological environment - extracellular matrix (e.g., collagen type 1), cell population (type A and B synoviocytes), and physiology (lining and sublining layer). A prerequisite is a comparative characterization of mesenchymal stromal cells (MSCs) with synovial fibroblasts from trauma patients ('healthy'), as MSCs are often used as fibroblastoid progenitor cells for in vitro modeling.
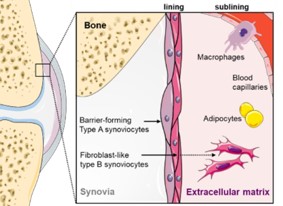
Fig. 1: The synovial membrane in vivo.
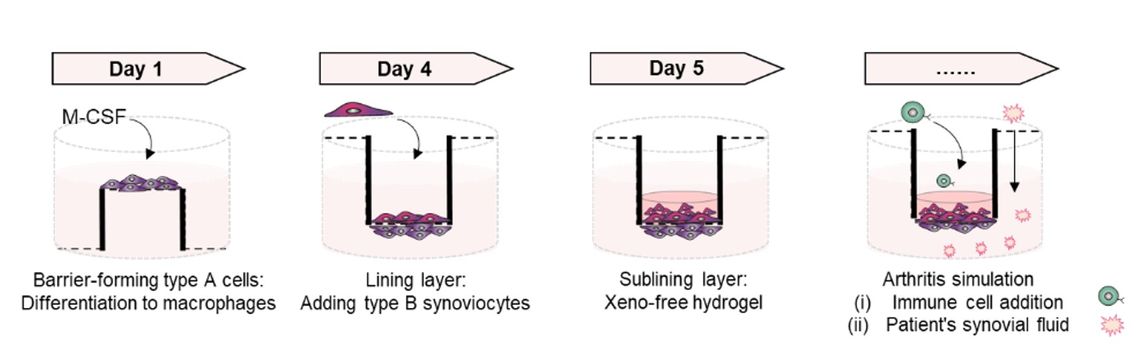
Fig. 2: Design of the in vitro 3D synovial membrane model..
Prospectively, the in vitro synovial membrane model offers an alternative to animal models in basic and biomedical research to (i) study pathophysiological processes, (ii) identify potential targets, (iii) test novel therapeutic approaches and biologics, and finally, (iv) reduce animal experiments.
Charité-Universitätsmedizin Berlin
Department of Rheumatology and Clinical Immunology
Charitéplatz 1
10117 Berlin, Germany
03/2022 – 02/2024