Fig. 1: Foamy macrophage phenotype following drug exposure.
A significant number of new compounds developed as oral inhaled products for the treatment of lung diseases show ambiguous macrophage effects during the early preclinical development stage. One of the most common effects is the accumulation of foamy macrophages at focal points in the lung, which may or may not be accompanied by inflammatory parameters.
The term “foamy macrophage” is used by pathologists to describe highly vacuolated macrophages observed in, not only the lung, but other organs as well, in the course of some diseases or drug treatments (Figure 1). They may develop as a result of different physiological or pathophysiological mechanisms, e.g. drug-induced phospholipidosis. Currently, it is postulated that vacuolated macrophages may also be induced through phagocytosis of poorly soluble inhaled drug particles, which may lead to a temporary increase in vacuolated macrophages in the lung during drug treatment. It remains unclear, whether foamy macrophages play a role in pathological processes or constitute a non-adverse response to inhaled poorly soluble matter. Due to this uncertainty, drugs that induce foamy macrophages in non-clinical studies may be withdrawn from the development process.
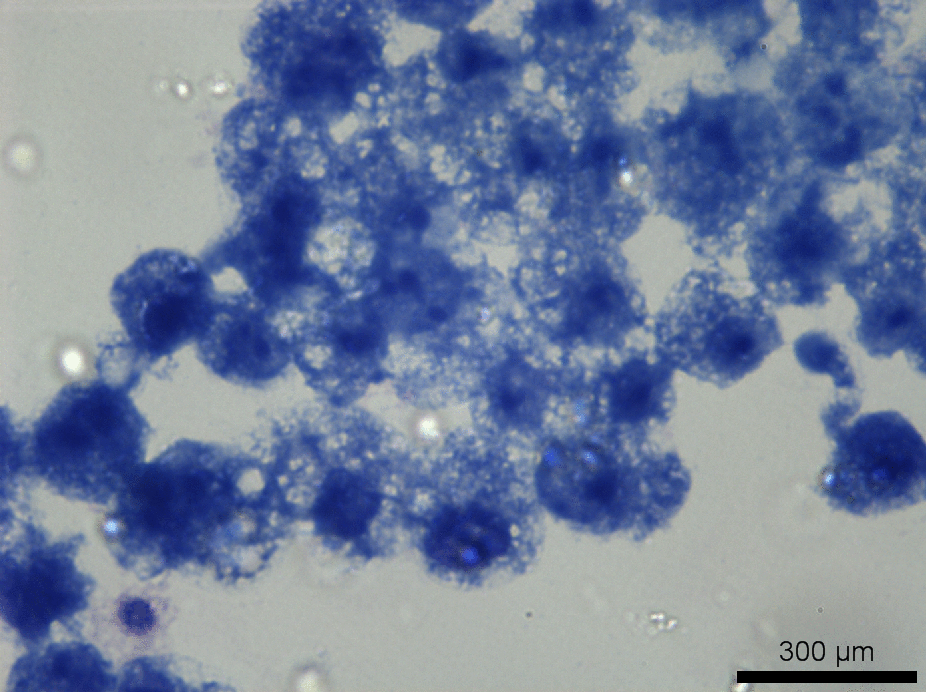
Fig. 1: Foamy macrophage phenotype following drug exposure.
A better understanding of foamy macrophage biology is required before it is possible to determine whether their appearance in non-clinical studies constitutes an adverse toxicological response. An in vitro assay for the identification and differentiation of drug-induced macrophage responses would enable the pharmaceutical industry to screen new compounds for a foamy macrophage phenotype prior to animal testing, thereby reducing the number of animal experiments performed on potentially problematic compounds.
In previous work, a multi-parameter assay, referred to here as a FoMac assay (“foamy macrophages”) has been developed to assess macrophage effects following exposure to different pharmaceutical compounds. Using a fluorescence imaging-based high content analysis approach, different cellular parameters, such as cell morphology, metabolic status, vacuolation and lipid content, can be assessed simultaneously. Compounds which induce vacuolation can be identified and assessed next to other potentially pathological effects (Figure 2).
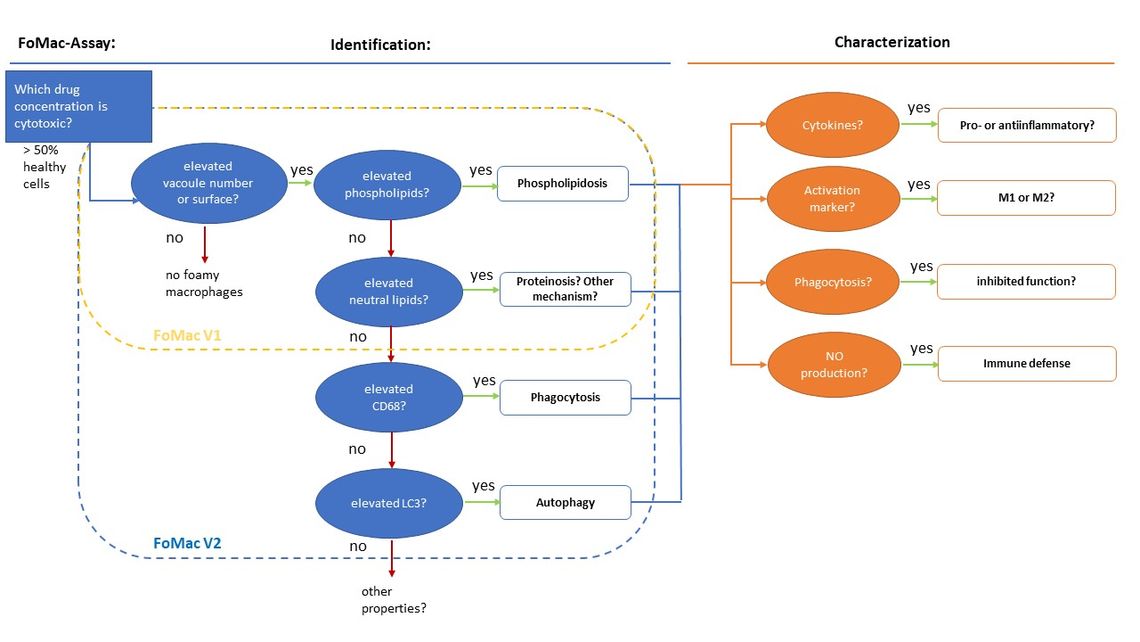
Fig. 2: Outline of the investigational parameters of the FoMac assay to identify and characterise foamy macrophages.
The instrumentation used in the FoMac assay is already widely established in the pharmaceutical industry, thus enabling a rapid integration of the screen into preclinical development without extra investment costs. Through the inclusion of further fluorescent markers in the assay, it may also be easily expanded to include a wide range of relevant biomarkers, which allows us to investigate questions arising about foamy macrophage biology beyond the basic assay set up.
Martin-Luther-Universität Halle-Wittenberg (MLU)
Institut für Pharmazeutische Technologie und Biopharmazie
Wolfgang-Langenbeck-Str. 4, D-06108 Halle (Saale)
02/2018 - 01/2019