The phenomenon of retinal degeneration occurs in many eye diseases, like glaucoma, Retinitis pigmentosa or retinal ischemia. Since the formation of these diseases is not fully understood yet, reliable models are needed to determine the pathological alterations. To date, these studies were based on acute disease induced animal models, which are bred and killed just for these experiments. Organotypic cultures of retinal explants, obtained from eyes from pigs slaughtered for the food industry, offer a good alternative. Retinal organ cultures are in-vitro-models with a high significance, since the cells are still in their natural cell-organization. In addition, the pig eye is more similar to the human eye in size and morphology than the most common laboratory animals, like rats, mice and rabbits. The goal of this project is to modify this model through the use of in-vivo stressors so it can be used as a screening-model for novel therapy options.
During this project the effect of three stressors on the loss of neuronal cells in the retina, especially ganglion cells, will be evaluated. Therefore, retinal explants, reproducible in size and shape, will be analyzed histologically in regard to possible alterations of ganglion cells but also the other structures of the retina are evaluated.
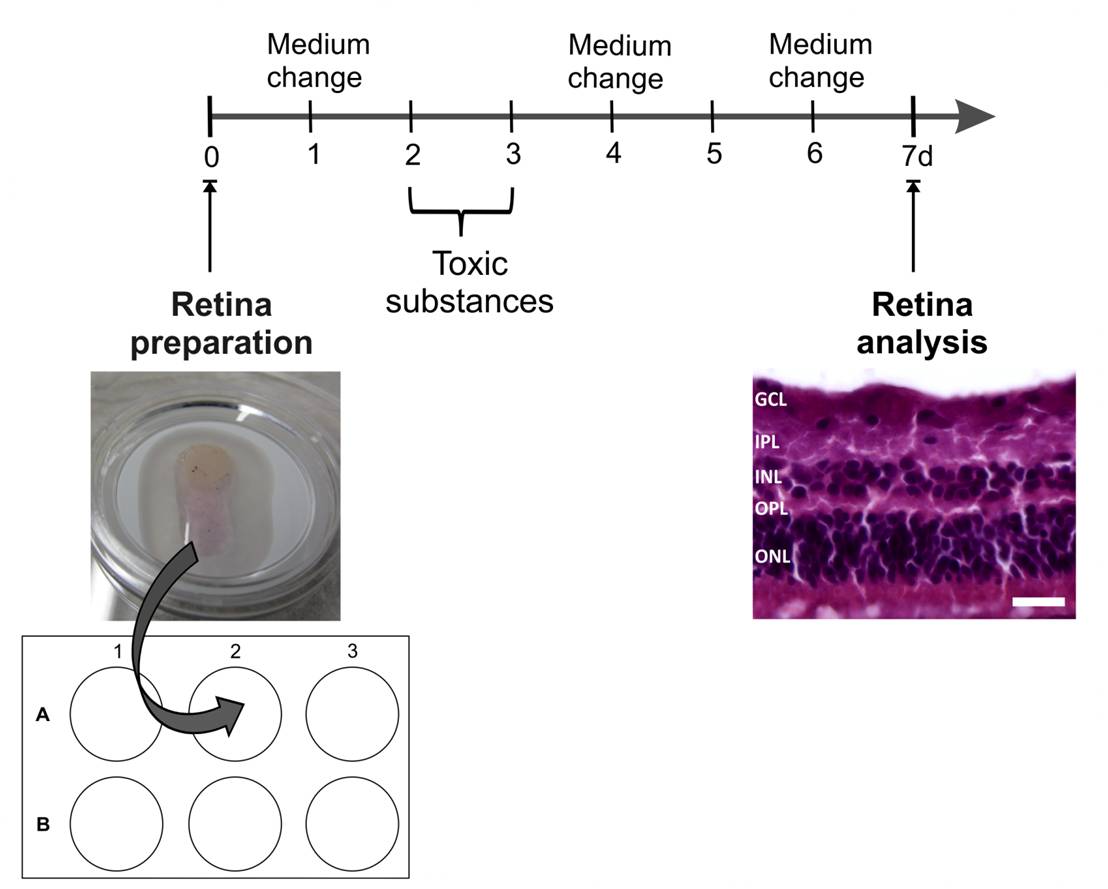
Fig. 1: Time-line of stressor treatment:
At day 0 the retinas will be dissected from the pig eyes, placed on an insert and cultured in a 6-well plate. At day one, four and six the medium will be changed. At day two and three the retinal explants will be treated with the toxic substance (stressor). The cultivation phase ends at day seven, when the explants are cry-conserved, to analyze the structures using quantitative real-time PCR, Western-blot or histology later on. The first evaluation is a hematoxylin and eosin stain of the retinal cross-sections to visualize possible alterations in the structure (Abbreviations: GCL = ganglion cell layer; IPL = inner plexiform layer; INL = inner nuclear layer; OPL = outer plexiform layer; ONL = outer nuclear layer; scale bar = 20 µm).
Apoptotic and immunologic processes play an important role during degeneration. To quantify the alterations of the different processes, like caspase-pathway or glia cells, they will be analyzed using quantitative real-time PCR on mRNA level and using Western-blot on protein level. The localization of the cell alterations in the retina is possible with histological evaluation. When the damage patterns and alterations induced by the stressors are known the optimal stressor-dose for the therapy-studies can be determined. During the therapy-studies two possible neuroprotectants will be tested against these stressors. The same methods as described before will be used for these studies. The RNA-expression (quantitative real-time PCR) and the protein-expression (histology and Western-blot) will be determined, to find out how they are altered by the stressors. This way, the effect of the neuroprotectants on the degenerating retinas can be evaluated.
This model can be seen as a model-system for pathological alterations during retinal diseases, e.g. glaucoma. It can be used for future studies testing therapeutic substances and for studies analyzing pathomechnisms or mode of actions and therefore contribute to lower numbers of research animals. This model can therefore replace animal experiments (Replace). Based on the size of the pig eyes they have the great advantage that four comparable samples can be taken from one retina. Using mice or rats the size of the eye and therefore the volume of the material (protein, DNA and RNA) would only be sufficient for one sample. The number of animals can, compared to mice and rats, be reduced by factor four (Reduce). Through the use of a porcine organ-culture-model for screening of therapeutic substances the doses of these substances can be optimized, before they are used in an animal model in-vivo (Refine).
Institutions
Research lab, University Eye Clinic, Knappschaftskrankenhaus, Ruhr-University Bochum
In der Schornau 23-25, 44892 Bochum
Research lab, University Eye Clinic Tübingen
Elfriede-Aulhorn-Str. 7, 72076 Tübingen
Duration
07/2014 - 06/2016

 English
English