Fig. 1: Experimental design and characteristic spectra of vaccines with different active components and their discrimination using machine learning methods (Kamp et al. 2021).
Vaccines against diphtheria, tetanus and pertussis (DTaP vaccines) are part of the standard vaccination schedule in humans both for children and adults. Tetanus vaccination also plays an important role in the veterinary sector. Within the framework of the federal batch release testing, these vaccines are monitored in animal experiments at the Paul Ehrlich Institute (PEI) in accordance with the specifications set by the European Pharmacopoeia. Serological tests are used to measure the necessary immune response in guinea pigs. Alternative methods of drug testing and quality control are the prerequisite for reducing and eventually replacing these animal tests.
Previous research at the PEI was able to highlight the potential of Raman spectroscopy for the investigation of vaccines (Silge et al. 2018; Kamp et al. 2021) and provides the basis for this project: vaccines can be reliably distinguished with the help of spectroscopy and machine learning methods and components contained therein can be identified. However, especially in the identification of the components responsible for the immune response, there are still challenges in the experimental execution of the measurement and the statistical analysis. DTP vaccines are complex biomedicines with a heterogeneous mixture of active components (proteins), potency enhancing adjuvants (crystalline or amorphous, inorganic substances), excipients (water) as well as stabilizers. Even more than with homogeneous chemical-pharmaceutical substances, well defined and controlled protocols are required to acquire reproducible and interpretable spectra.
We are developing these protocols with a special focus on the active components of vaccines. Machine learning methods already allow to reliably distinguish vaccine spectra, and we are now developing these methods further to be able to reliably identify and quantify active components. In the case of DTaP vaccines, we initially focus on the tetanus active component and develop a spectroscopic method of quality control.
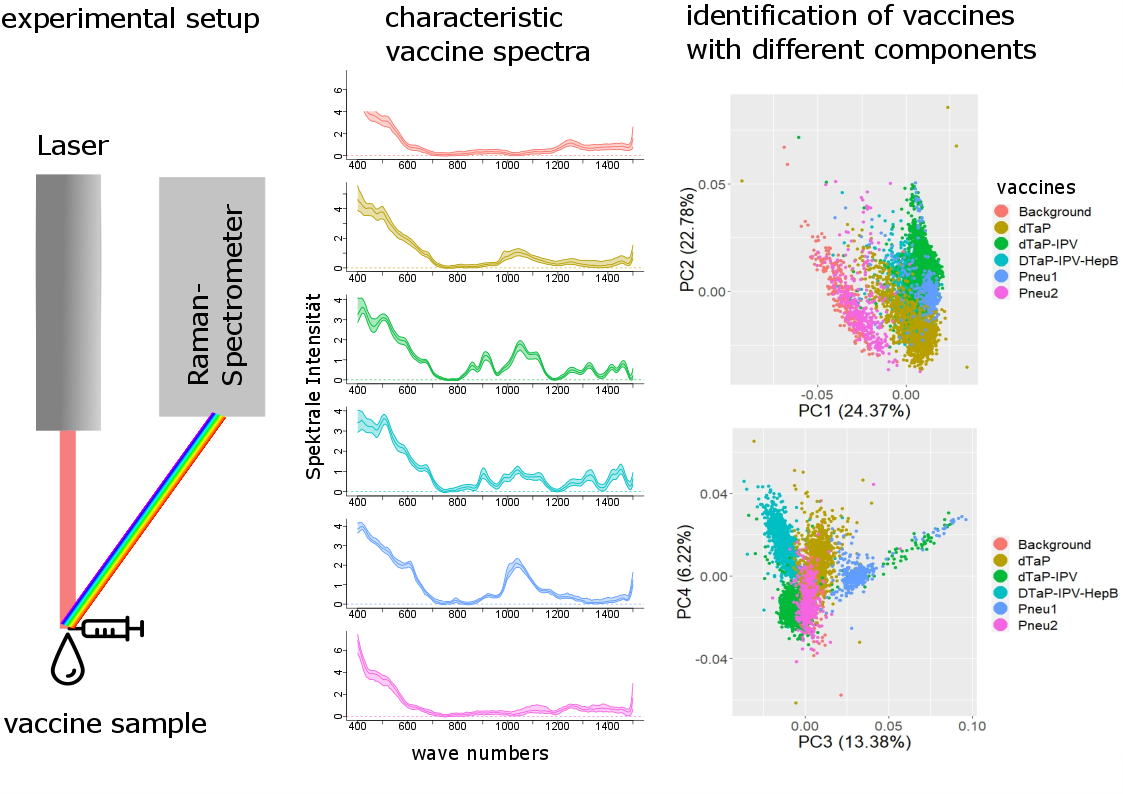
Fig. 1: Experimental design and characteristic spectra of vaccines with different active components and their discrimination using machine learning methods (Kamp et al. 2021).
In this way, we are opening up the prospect of the method being included in the European Pharmacopoeia as an alternative method for the currently mandatory animal testing. Through our active participation in the committees of the Pharmacopoeia Commission, we are helping to ensure that once the method has been successfully established, its widespread use in practice can also become a reality.
Publications:
Silge, A., Bocklitz, T., Becker, B., Matheis, W., Popp, J., Bekeredjian-Ding, I. “Raman spectroscopy-based identification of toxoid vaccine product” npj Vaccines 3, 50 (2018). doi.org/10.1038/s41541-018-0088-y.
Kamp, C., Becker, B., Matheis, W., Öppling, V., Bekeredjian-Ding, I. "How to draw the line – Raman spectroscopy as a tool for the assessment of biomedicines" Biological Chemistry, vol. 402, no. 8, pp. 1001-1006 (2021). doi.org/10.1515/hsz-2020-0388.
Paul-Ehrlich-Institut
Federal Institute for Vaccines and Biomedicines
Paul-Ehrlich-Str. 51-59, 63225 Langen, Germany
01/2023 - 12/2024