Fig. 1: Structure of the tumor model.
Neuroblastoma cells (IMR-32) are surrounded by renal cells. Initially HEK293 or HEK293-GFP cells are used for this purpose, in later experiments also primary kidney fibroblasts.
The transferability of results from animal experiments to humans is particularly poor in the field of cancer research. Around 97% of all oncology drug candidates that have proven to be efficient and safe in preclinical studies in animal models fail in human clinical trials. One important reason for the low chance of success is the species-specific differences between animals and humans. At the same time, in vivo trials in cancer research are particularly stressful for the animals.
In the funded project, therefore, human tumor models are to be generated by means of bioprinting. In this process, a tumor is embedded in a tumor microenvironment consisting of healthy human cells (Fig. 1).
Fig. 1: Structure of the tumor model.
Neuroblastoma cells (IMR-32) are surrounded by renal cells. Initially HEK293 or HEK293-GFP cells are used for this purpose, in later experiments also primary kidney fibroblasts.
This allows to study the very important interactions between the tumor and its surrounding cells. As an example, a tumor consisting of neuroblastoma cells surrounded by kidney cells will be printed. The kidney is an occasional, albeit rare, site for metastases from neuroblastoma.
In a further step, the tumor model is treated with various substances. In preliminary experiments, we have already shown that the anticancer drug panobinostat selectively kills the cancer cells by inducing apoptosis, while the healthy cells of the tumor environment remain unaffected (Fig. 2).
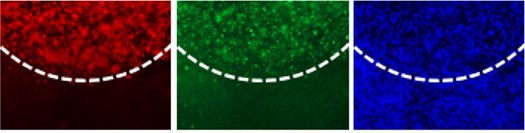
Fig. 2: Fluorescence microscopic analysis of the tumor model after treatment with an anticancer drug (panobinostat).
The red fluorescence is from a marker (GD2) for the neuroblastoma cells. These cells go into programmed cell death (apoptosis) after treatment with panobinostat. The apoptosis marker cleaved caspase-3 was stained in green. The blue staining of the cell nuclei indicates the distribution of all cells.
In order to reproduce the natural physiology of tissues, it is important to vascularize the in vitro organ models and perfuse them with a media flow. Here, bioprinting offers the possibility of integrating channel structures, which represent natural blood vessels, into the 3D constructs. As part of the project, a channel was successfully implemented in a model of a lung tumor. The model was connected to a media stream (Fig. 3). This increased the viability of the cells, and a tested cancer drug worked better under these dynamic conditions than under static cultivation.
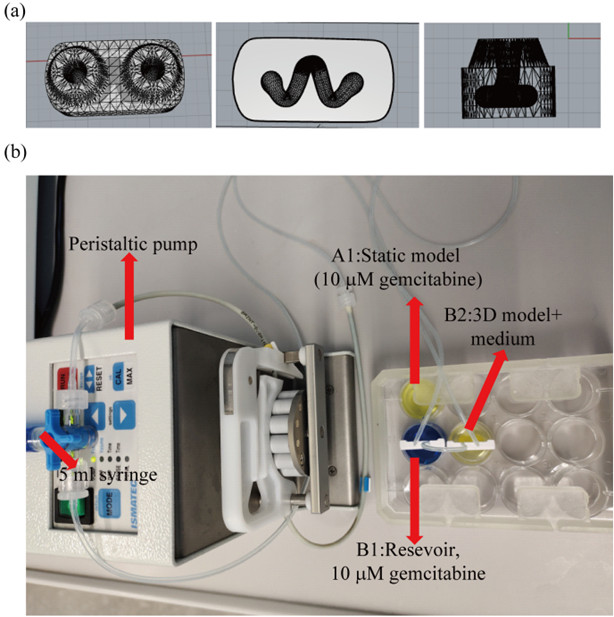
Fig. 3:
Design of the printed lung tumor model with a channel running through it (a). The organ model was connected to a peristaltic pump to flush it with a media flow (b).
A further developed version of the tumor model consists of a cancerous core located in an environment of healthy cells. This model can be used to study both, the efficiency of new cancer drugs and their side effects on surrounding tissue.
Publikations:
Wu, D.; Berg, J.; Arlt, B.; Röhrs, V.; Al-Zeer, M.A.; Deubzer, H.E.; Kurreck, J. Bioprinted Cancer Model of Neuroblastoma in a Renal Microenvironment as an Efficiently Applicable Drug Testing Platform. Int. J. Mol. Sci. 2022, 23, 122. https:// doi.org/10.3390/ijms23010122
Mei, Y.; Wu, D.; Berg, J.; Tolksdorf, B.; Roehrs, V.; Kurreck, A.; Hiller, T.; Kurreck, J., Generation of a Perfusable 3D Lung Cancer Model by Digital Light Processing. Int. J. Mol. Sci. 2023, 24, 6071.
Wu, D.; Pang, S.; Berg, J.; Mei, Y., Ali, A.S.M.; Röhrs, V.; Tolksdorf, B.; Hagenbuchner, J.; Ausserlechner, M. J.; Deubzer, H.E.; Gurlo, A.; Kurreck, J. Bioprinting of perfusable vascularized organ models for drug development via sacrificial-free direct ink writing. (Submitted)
Prof. Dr. Jens Kurreck
Technische Universität Berlin
Institut für Biotechnologie, Fachgebiet Angewandte Biochemie, TIB 4/3-2
Gustav-Meyer-Allee 25
13355 Berlin, Germany
10/2021 - 09/2023