Inhaled particles or fibres may cause acute inflammation of the lung or, in some cases, may lead to delayed effects such as lung fibrosis or cancer. Particles or dusts which enter the deep regions of the lung parenchyma due to their small size of less than 5 µm are of relevance for occupational health, and need to be tested toxicologically. This is routinely carried out by an inhalation toxicology approach according to OECD guidelines. About 50,000 rats are subjected to this testing strategy every year. With the advent of the REACH legislation and the adaptation of the guidelines to nanoparticles a further increase of this number is to be expected in Europe over the next years.
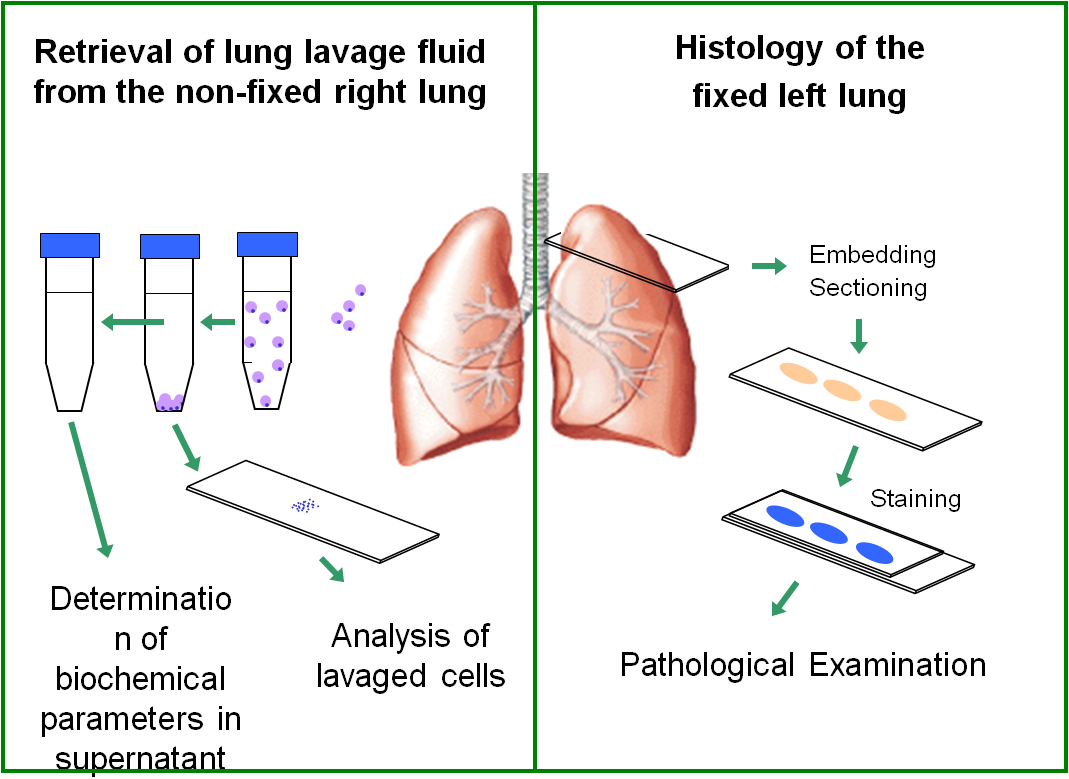
During particle exposure the experimental animals (rats) are exposed to particle-enriched air (mostly <50 mg/m3). To make sure that particles are exclusively taken up via inhalation, rats are placed in tight tubes from which only the animals' snout protrude into the particle containing atmosphere. This situation is maintained for six hours a day and repeated daily for several weeks, with the overall time depending on the design of the study. To assess the biological effects of particles, the broncho-alveolar lavage fluid (BALF) as well as the tissue structure of the lung is investigated at various points in time secondary to particle exposure. Invaded inflammatory cells and other components of the BALF, as well as possible alterations of the lung tissue are analysed. Figures 1 and 2 summarize the major steps of this process and show some typical results. Most importantly, only the synopsis of all results allows for a sound interpretation of particle effects on lung tissue. Therefore, wo animal collectives were required to separately study effects on histology and BALF parameters.
The project aimed at carrying out these investigations on only one animal collective. To achieve this goal the left lung should be used for histology only, whereas the right lung should be used for BALF preparation. This Hybrid Preparation had not yet been used for toxicological routine testing due to some intricate and time-consuming steps. Moreover, no systematically conducted studies were available to compare the outcome of both methods.
In the project the methodology should be optimised such that it might become implemented into existing protocols. This was to be achieved in conjunction with the experienced animal testing laboratory of BASF-SE. In parallel, the urgently needed data for comparing both methods were to be generated. In case the new Hybrid Preparation would turn out to be sufficient for evaluating particle effects on the lung, it should be proposed to OECD with the aim to become part of an improved guideline.
The Hybrid Preparation has a strong relation to the 3R concept: Although it is not an in vitro method meant to mimic the complexity of the lung and to replace the animal experiment, the method has a strong potential to reduce animal numbers by half. Bearing in mind the expected increment in animal numbers as outlined above, this reduction appears highly desirable. Moreover, data for each animal will be more complete and this, in the sense of an refinement, will ease evaluation in case of uncertainty or inter-individual difference.
Fig. 1: Application of the Hybrid Preparation allows to retrieve broncho-alveolar lavage fluid from the right lung only, while the left lung is used for histology. In the currently used methodology two separate animal collectives are used for these investigations.
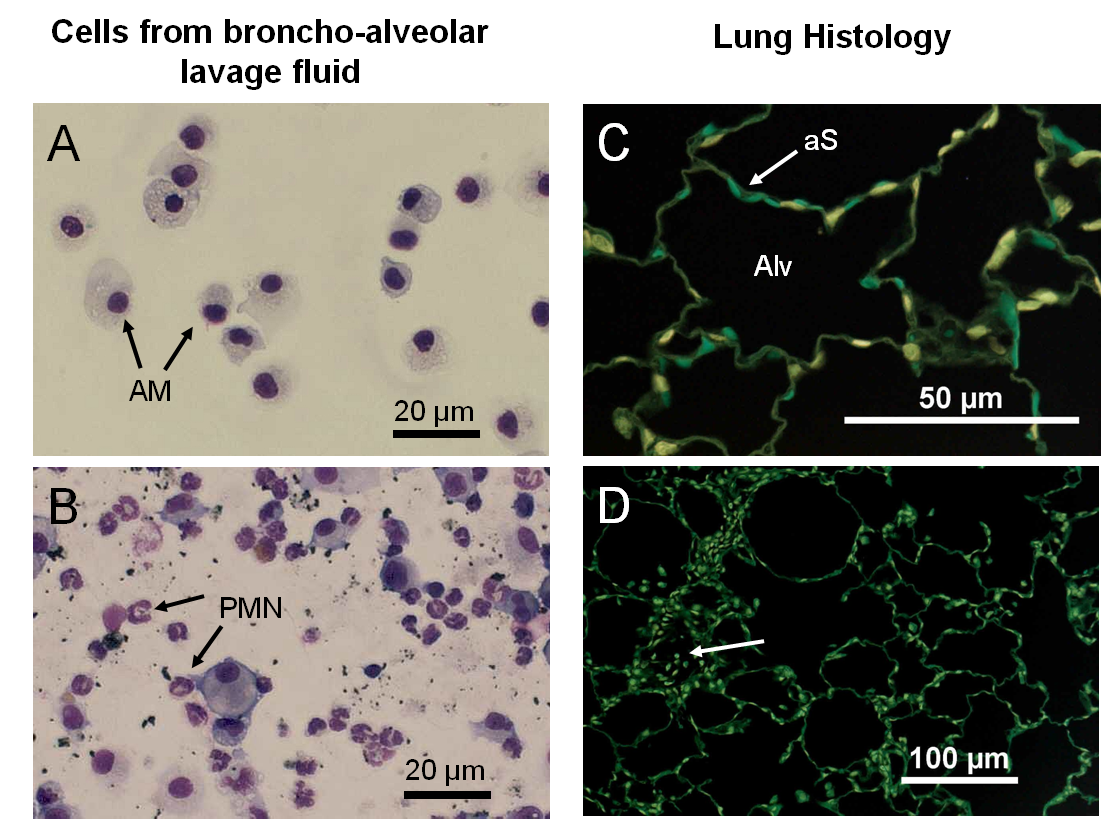
Fig. 2: Micropgraphs from typical broncho-alveolar lavage fluids (A, B) and lung tissue sections (C, D). Alveolar macrophages (AM) are the dominant immune cells lavaged from a healthy lung (A). Particle load leads to signs of inflammation (B) indicated by numerous neutrophilic granulocytes (PMN). Lung histology of a healthy lung (C) shows air-filled alveoli (Alv) formed by the fine alveolar septa (aS). In particle-laden lungs (D) cells can be found inside alveoli (arrow). Moreover, growth processes may lead e.g. to a thickening of the alveolar septa and diminished gas transport capabilities. (A, B) May-Grünwald-Giemsa staining, (C, D) paraffin sections, HE-staining, digitally inverted.
Results:
The project was finalized successfully. According to some changes and modification of the planned experiments we succeeded in preparing the lungs of experimental animals (rats) such that both broncho-alveolar lavage fluid and lung histology could be assessed without limitations from the same animal. In the sense of the 3R principle the method therefore allows to reduce the number of animals for this type of tests by half. Furthermore it comes along with a refinement as more data may be gathered from each animal which is always benefical in case of inter-individual variation.
Various trials were necessary to achieve the success of the project. First experiments were carried out with healthy and quartz particle-subjected rats, to consider the influence of inflammatory changes on the new procedure. In both groups of animals the right lung was subjected to a manual lavage procedure during which the lung tissue is repeatedly flooded with fluid and simultaneously massaged to increase the yield of washed-out immune cells for further tests. For either group of animals it could be shown that the unilateral lavage had no disadvantage compared to the bilateral approach in terms of quantitative evaluation of inflammatory parameters. Further experiments carried out in collaboration with animal pathologists from BASF confirmed these results and showed that the non-lavaged left lung was in fact well suited for routine pathology. Moreover, the histology of the lavaged right lung was in surprisingly good condition despite previous lavage and all further steps of manual manipulation. This is an important result because the right lung consists of four so-called lobes each of which may still be evaluated histologically. Small or locally confined alterations of the lung tissue, such as e.g. beginning tumour growth, should therefore be discoverable as good and reliable as before. So there is no need to fear that any sign of lung tissue alteration may be overlooked.
A direct effect of this project was the revision of the OECD test guideline 413 (TG413). Now Hybrid Preparation is the standard method for subchronic inhalation toxicity studies for nanoparticles in rats.
Institution
IBE R&D gGmbH Institute for Lung Health
Mendelstr. 11, D-48149 Münster
Project Partner
Dr. Robert Landsiedel
BASF Product Safety - Experimental Toxicology and Ecology
BASF-SE, GV/TB - Z470, 67056 Ludwigshafen
Duration
06/2013 - 12/2013

 English
English