Fig. 1:
Schematic illustration of the standardized and controlled induction of manifest tissue infections including biofilm formation using the developed in vitro biofilm platform model.
About 80% of all chronic tissue infections are estimated to be related to the presence of bacterial biofilms. Due to a pronounced tolerance of biofilms against antimicrobial actives, those infections account for complicated therapies and high burden for the patients in a clinical setting. Thus, reliable models for investigating the development and therapy of biofilm-related infections are urgently needed.
So far, research in this field mainly bases on animal models. However, reduced translatability to the human system as well as particular ethical concerns highlight the need for a robust and versatile in vitro model of bacterial biofilms as a valid alternative to animal testing. Established in vitro biofilm models based on flat, abiotic surfaces do usually not include human cells or tissues, resulting in a reduced clinical relevance.
In this study we developed a biofilm model based on three-dimensional scaffolds of electrospun fibers, aiming at a close imitation of the microenvironment of native biofilms and a transfer to human tissue models upon biofilm maturation.
Via electrospinning fibers out of biopolymers were fabricated and their diameter was adapted to the size of relevant pathogens of tissue infections. In combination with an appropriate wettability, this fiber characteristic should facilitate bacterial adhesion to the scaffolds. Furthermore, the fiber scaffolds were designed to provide high mechanical stability in order to enable a later transfer of the biofilm models. A successful colonization by one or multiple bacterial strains (e.g., Pseudomonas aeruginosa, Staphylococcus aureus) was further demonstrated, resulting in bacterial adhesion, colony formation and the development of mature biofilms, which provided key characteristics of native biofilms present in tissue infections. In a next step, human-based wound models were infected with the separately cultivated biofilm-model to simulate a chronic state of wound infection without long-term damage of the tissue (Figure 1).
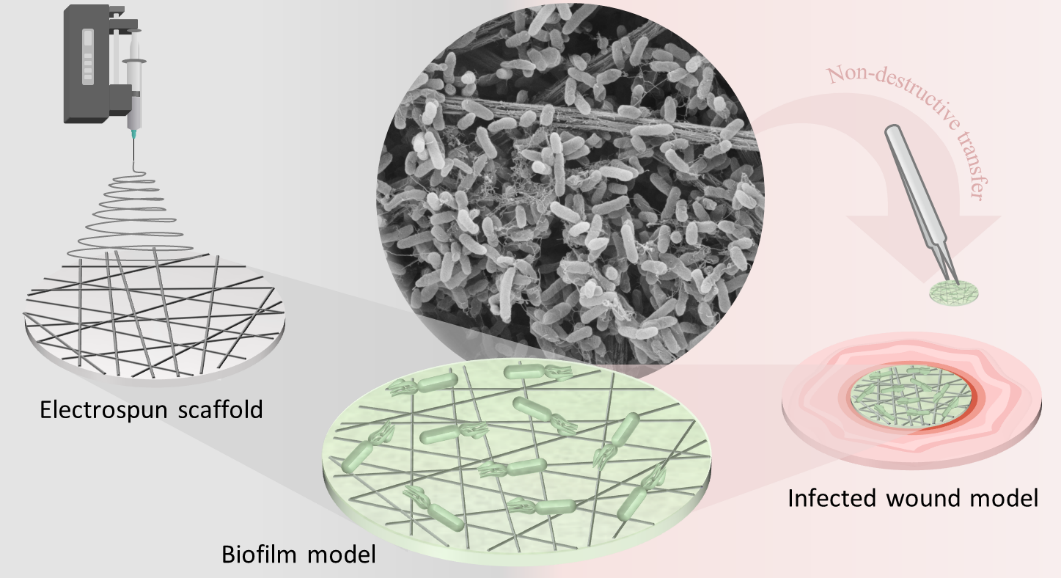
Fig. 1:
Schematic illustration of the standardized and controlled induction of manifest tissue infections including biofilm formation using the developed in vitro biofilm platform model.
The developed biofilm model closely reflects the in vivo situation of biofilm-related tissue infections, resulting in a high clinical relevance. This opens the opportunity to study pathological mechanisms of a manifest bacterial infection in vitro and to develop new approaches for effective therapies.
Publikationen:
Waechter J, Vestweber PK, Jung N & Windbergs M, 2023. Imitating the microenvironment of native biofilms using nanofibrous scaffolds to emulate chronic wound infections. J. Mater. Chem. B, 2023, 11, 3212
Waechter J, Vestweber PK, Planz V & Windbergs M, 2023. Unravelling host-pathogen interactions by biofilm infected human wound models. Biofilm 6 (2023) 100164
Research group Prof. Dr. Maike Windbergs “Drug Delivery and 3R-Models”
Goethe University Frankfurt
Institute for Pharmaceutical Technology and Buchmann Institute of Molecular Life Sciences
Max-von-Laue-Str. 9, 60438 Frankfurt am Main
05/2020 - 10/2022