Fig. 1: iPSC-derived podocytes are growing on either Matrigel (A) or on different Matrigel-free coatings (B-D).
Human cell culture systems play an important role in research, for example in toxicology, where they are used to understand the mechanisms of actions of medicines or toxins. In particular so called human induced pluripotent stem cells (iPSC) are expected to be of great importance in the development of more relevant, animal-free test systems. iPSC can be generated from adult cells, e.g., blood cells, and in theory can be differentiated into any cell type of the body. In our laboratory, we have differentiated iPSC into different cells of the kidney, including podocytes, to test compounds for renal toxicity.
However, for culturing iPSC, a coating made of a specific extracellular matrix is necessary that allows the cells to attach to the culture dish. For this, a product called Matrigel is widely used. Matrigel is an animal product and for its production mice have to be induced with sarcoma tumours. The weight of these tumours often reaches up to 20% of total body weight of the mice before they are finally sacrificed.
While Matrigel is one of the most commonly used coatings for culturing iPSC, its replacement is desired for several reasons, including better standardization conditions and of course for ethical reasons. Even though some alternative products are commercially available, these are only used sparely in laboratories. Reasons for this vary and include often higher costs of alternative products and limited independent studies that compare different products from different companies.
In this project one of our aims was to carefully evaluate and compare existing products, with a major focus on iPSC quality and stability: iPSC have to remain pluripotent and should not acquire genetic variations. Furthermore, we were working on the development of new and more cost-effective coatings by employing human cell lines.
The third aim of this project was to test these coatings for the differentiation of iPSC into renal cell types (e.g. podocytes) in order to develop a new differentiation protocol that is independent from Matrigel. Here, we analysed podocyte specific markers, including synaptopodin and WT1.
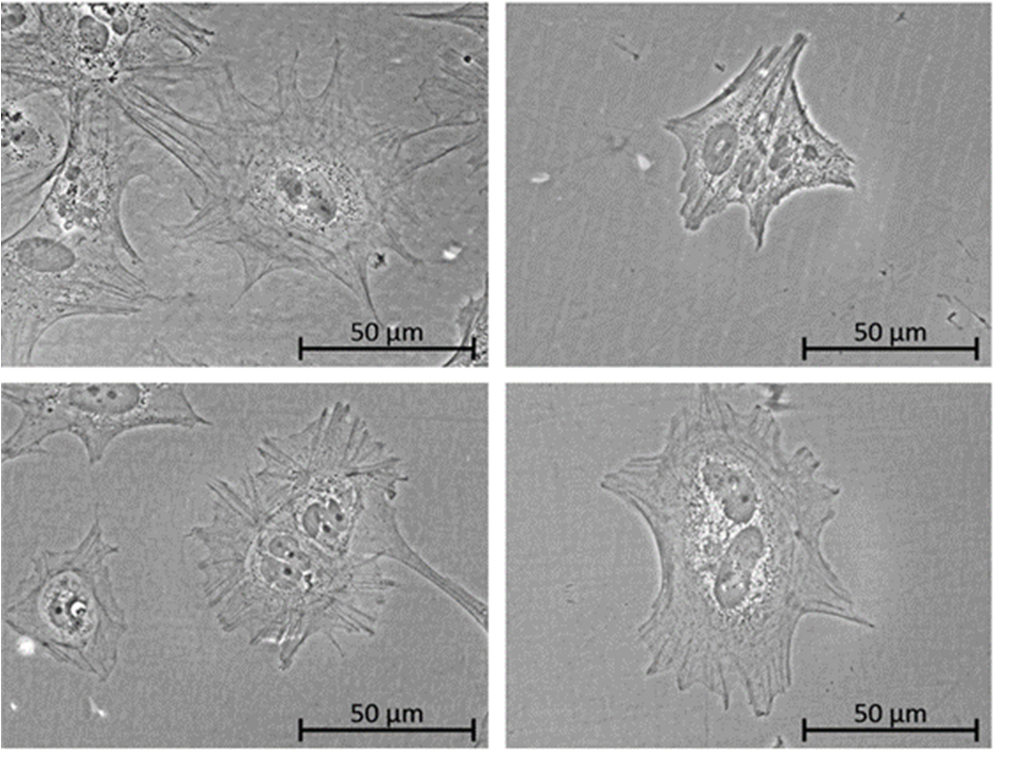
Fig. 1: iPSC-derived podocytes are growing on either Matrigel (A) or on different Matrigel-free coatings (B-D).
The project was successfully completed. It showed that iPSC maintenance could be accomplished on the human recombinant protein coatings vitronectin, laminin-511 and laminin-521 as well as on the human fibroblast-derived ECM coating. Undifferentiated iPSC (of 3 different donors) showed similar pluripotency markers when compared to cells cultured on animal-derived Matrigel. Furthermore, the karyotype stability and the differentiation potential into the three germ layers was comparable on all tested coatings.
Differentiation of iPSC into podocyte-like cells (of 3 donors) could also be accomplished on the human recombinant coatings vitronectin, laminin-511 and laminin-521 and the human fibroblast-derived ECM coating and results were similar to differentiations on Matrigel. In addition, we also tested differentiation of iPSC in renal proximal-like cells (PTL) (1 donor). Here, the harvested ECM from human fibroblasts or from human renal cells (RPTEC/TERT1) showed best results that were as good as when differentiations were performed on Matrigel. The human recombinant coatings vitronectin and laminin-511 did not work as well in this case.
We could conclude that Matrigel is not needed for iPSC maintenance and differentiation into renal podocytes and proximal tubular cells. Alternative coatings are available that have now been extensively evaluated. The results have been published in ALTEX (2022):
Publications:
Murphy, C., Naderlinger, E., Mater, A., Kluin, R. J. C. and Wilmes, A. (2023) “Comparison of human recombinant protein coatings and fibroblast-ECM to Matrigel for induced pluripotent stem cell culture and renal podocyte differentiation”, ALTEX - Alternatives to animal experimentation, 40(1), pp. 141–159. doi: 10.14573/altex.2112204.
Chandrasekaran, V., Carta, G., da Costa Pereira, D. et al. (2021). “Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability”. Scientific Reports 11, 11575. doi.org/10.1038/s41598-021-89550-4.
Division of Molecular and Computational Toxicology
Vrije Universiteit Amsterdam,
De Boelelaan, 1108,
1081 HZ Amsterdam, The Netherlands.
01/2020 - 12/2021