Fig. 1: Overview of the different methodological approaches used to assess nephrotoxicity in larval zebrafish exposed to model nephrotoxins.
The kidney is a major target organ of toxicity. Numerous structurally diverse drugs, natural compounds and chemicals can cause kidney injury. Presently, nephrotoxic effects of drugs and chemicals are identified primarily through regulatory toxicity tests in animals. With the aim of more efficient safety assessment and reduction of animal testing, toxicology is currently undergoing a paradigm shift in the way toxicity testing and risk assessment is carried out - away from tests in animals towards mechanism-based in vitro methods. While cell-based in vitro assays are particularly attractive for high-throughput screening, they fail to adequately reflect the complex processes that occur in vivo and thus do not allow prediction of potential health risks of drugs and chemicals with high confidence. Embryonic zebrafish (Danio rerio), which according to animal welfare legislation are considered as an in vitro model, are recognized as an alternative model for toxicity screening as they are easily accessible, releatively cheap and suitable for high-throuput methods, and - more importantly - better reflect the physiological situation of an intact organism (e.g. with respect to toxicokinetics, biotransformation) than cell-based in vitro assays.
Despite its relatively simple anatomical structure composed of only two nephrons, the anatomical organization (glomerulus, proximal tubule, distal tubule, collecting duct) and function of the pronephros in embryonic zebrafish closely resemble the human kidney (metanephros). Cell types, differentiation pathways and molecular signaling pathways are conserved between kidney of zebrafish and human. Key transporters (e.g. slc20a1a, sglt, slc13a3, slc4a4) and the endocytic receptor megalin are expressed in the respective nephron segments. Glomerular filtration starts within 48h post fertilization. Thus, embryonic zebrafish fulfil fundamental requirements for an alternative model for nephrotoxicity testing.
To explore if larval zebrafish presents a suitable model for identification of compounds with nephrotoxic potential, this project was designed to assess adverse effects of model nephrotoxins on the pronephros of zebrafish larvae. The selected model compounds included aristolochic acid, cadmium chloride, ochratoxin A, potassium bromate and gentamicin, which are well characterized nephrotoxins in rodents and are known to induce kidney injury via different modes of action. In contrast to studies focusing on developmental nephrotoxicity, larvae were exposed from day 3 post fertilization, i.e. when organogenesis is complete, until 5 days post fertilization, after which zebrafish larvae are no longer considered an in vitro model. Using a combination of histological, biochemical, molecular and imaging techniques to assess treatment related effects on pronephros structure and function (Figure 1), changes indicative of pronephros injury consistent with those observed in rodents were observed in response to 4 out of 5 model compounds.
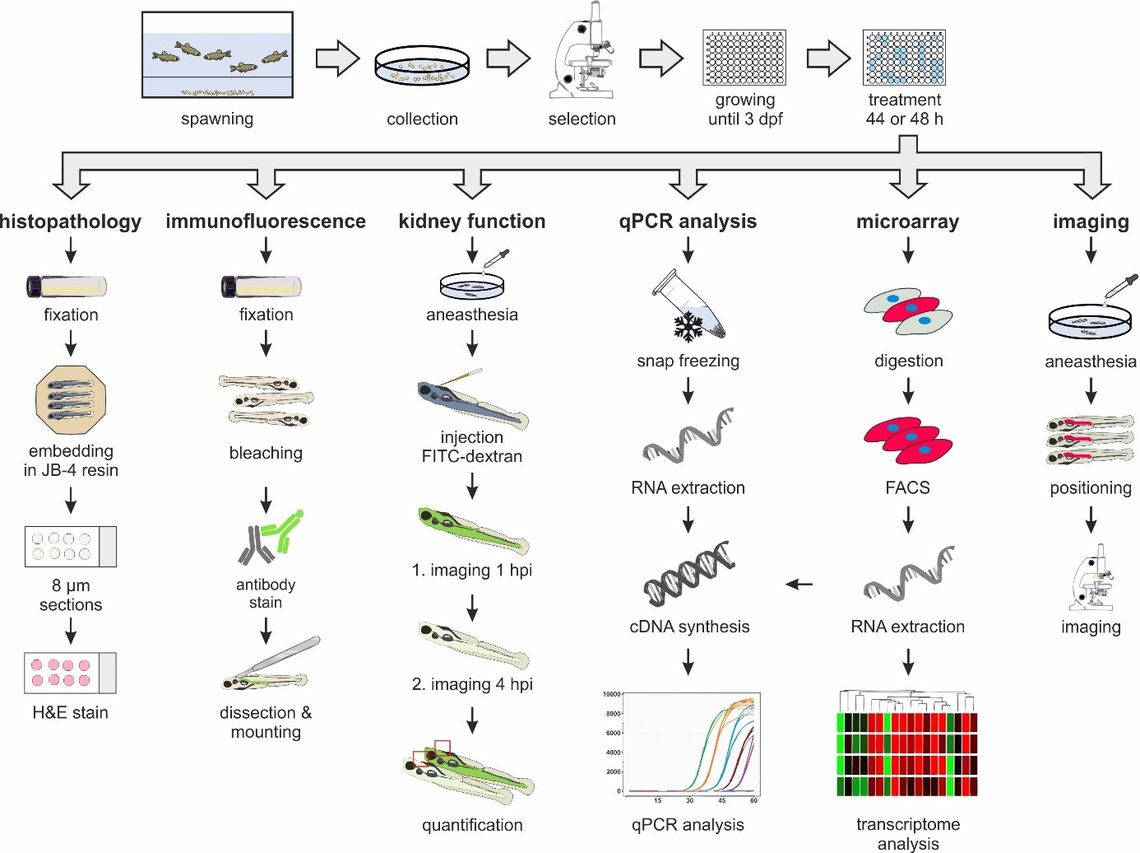
Fig. 1: Overview of the different methodological approaches used to assess nephrotoxicity in larval zebrafish exposed to model nephrotoxins.
In zebrafish larvae exposed to model nephrotoxins, marked histopathological changes were recorded in the proximal tubule of the pronephros. Pronephros injury was accompanied by altered expression of the pronephros-specific marker sodium-potassium-ATPase α6F and – in some cases - reduced renal clearance of FITC-dextran indicative of impaired kidney function. These results indicate that characteristic features of nephrotoxicity induced by model compounds in rodents can be reproduced in zebrafish larvae. The structural and functional changes were also associated with changes on the molecular level. A panel of kidney injury marker genes established as sensitive indicators of nephrotoxicity in the rodent model was found to be deregulated in pronephros cells isolated from larval zebrafish exposed to model nephrotoxins. These included heme oxygenase 1 (hmox1), connective tissue growth factor (ctgf), kidney injury molecule 1 (kim-1) and clusterin (clu), as well as the pronephros-specific marker gene cdh17. The value of cdh17 for visualizing pronephros injury was further confirmed by fluorescence imaging of a transparent cdh17:mCherry reporter fish line.
Considering the advantages of larval zebrafish (e.g. low costs, optical transparency, amenability to high-throughput screening), the results obtained indicate a great potential of this model for detection of nephrotoxicity. In particular, integration of the transparent cdh17: mCherry reporter line into an automated imaging pipeline may present a valuable approach towards establishing a high-throughput amenable assay for nephrotoxicity screening, which may accelerate the decision-making process during hit-to-lead screening of new compounds, ultimately leading to a reduction of animal experiments. However, understanding of the toxicokinetics of nephrotoxic compounds in zebrafish larvae and extrapolation of effective concentrations in larval zebrafish to human relevant doses is yet needed in order to derive safe doses for regulatory decision making.
Publikations:
Bauer, B., Liedtke, D., Jarzina, S., Stammler, E., Kreisel, K., Lalomia, V., Diefenbacher, M., Klopocki, E., Mally, A., 2021. Exploration of zebrafish larvae as an alternative whole-animal model for nephrotoxicity testing. Toxicol Lett 344, 69-81.
Bauer, B., Stammler, E., Liedtke, D., Mally, A. Characterization of site-specific nephrotoxicity in embryonic zebrafish. Naunyn-Schmiedeberg's Arch Pharmacol (2019) 39 (Suppl1): S80
Bauer, B., Liedtke, D., Stammler, E., Kreisel, K., Lalomia, V., Kruse, M., Diefenbacher, M., Klopocki, E., Mally, A. Evaluation of larval zebrafish as an alternative whole-animal model for nephrotoxicity testing. EUROTOX Congress 2021
Bachelor thesis Viola Lalomia, 2020: „Untersuchen der Nephrotoxizität in Larven des Zebrabärblings durch Genexpressionsanalyse“
Bachelor thesis Marlien Kruse, 2020: „Untersuchungen zur potenziellen Hochdurchsatzfähigkeit transgener embryonaler Zebrabärblinge zur Testung auf Nephrotoxizität“
Group Prof. Dr. Angela Mally:
Institut für Pharmakologie und Toxikologie
Universität Würzburg
Versbacher Straße 9, 97078 Würzburg
Dr. Daniel Liedtke:
Institut für Humangenetik
Biozentrum
Universität Würzburg
97074 Würzburg
06/2018 - 02/2021